Performance Analysis
The approach taken to evaluate performance for homocysteine assays is the same as that employed for factor assays in the laboratory level 1& 2 programme, complying with specifications detailed in ISO 13528 Statistical Methods for use in proficiency testing by interlaboratory comparisons.
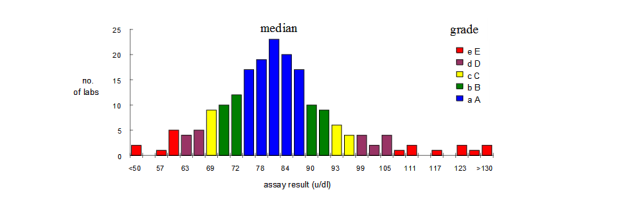
The overall consensus median is taken as the central reference point or "target value". Individual results are ranked into 5 unequal quantiles above and below the median, each quantile being designated by a letter depending on ranked distance from the median:
- A: The nearest 25% of results above (A) and below (a) the median (i.e. 50% of results)
- B: The next 10% of results above (B) and below (b) the median (i.e. 20% of results)
- C: The next 5% of results above (C) and below (c) the median (i.e. 10% of results)
- D: The next 5% of results above (D) and below (d) the median (i.e. 10% of results)
- E: The 5% of results furthest from the median, above (E) and below (e) (i.e.10% of results)
This is illustrated below:
Grades below the median are shown in lower case, and above the median in upper case, to aid in assessment of bias.
Performance is based on grades obtained in a minimum of two consecutive exercises for any particular test. "Outwith consensus" is defined as a combination of a C (or 'c') grade together with an E (or 'e') grade, or any combination of D (or 'd') and E (or 'e') grades (e.g. cE, ec, Dd, de, ED, and EE in consecutive distributions of that particular assay).
Persistent "outwith consensus" performance is defined as two consecutive "outwith consensus" performances, where the order in which the grades were assigned does not affect the overall performance. This will arise from three consecutive performances with the following combinations of grades (upper case only shown):
DDD, DED, ECE, EEC, DDE, DEE, EDD, EED, CEE, EDE, EEE
A non-return for a registered test will be graded as 'F' and taken as equivalent to an E grading. Thus, designations which include 'F' grades are based on performance over 2 or 3 exercises, respectively.
If results are persistently outwith consensus, a letter of concern with an offer of assistance is sent to the Head of Department by the Scheme Director. The Director will also make contact after a single survey for any results deemed to be sufficiently far from the target result to be considered clinically hazardous.
For homocysteine assays, we do not carry out separate analysis for different methodologies; however, we do not calculate method-specific median results, and these are shown on the survey reports.